CEIm
The Ethics Committee for Research with Medicines (CEIm) of the Salamanca Health Area is responsible for evaluating and supervising clinical trials, observational studies and other biomedical research studies in primary care and specialized care, all from the Complejo Asistencial Universitario de Salamanca. At IBSAL, these functions are applied to human studies.
What is its function?
The main function of the IRB/IEC is to report on the ethical, methodological and legal suitability of a biomedical study. This assessment is carried out before the start of the study, in order to approve its start-up, and throughout the course of the study.
This committee was accredited as CEIm by the Junta de Castilla y León on January 29, 2018, in accordance with the “Real Decreto 1090/2015, de 4 de diciembre, por el que se regulan los ensayos clínicos con medicamentos, los Comités de Ética de la Investigación con medicamentos y el Registro Español de Estudios Clínicos” (Royal Decree 1090/2015, of December 4, regulating clinical trials with medicinal products, the Ethics Committees for Research with medicinal products and the Spanish Registry of Clinical Studies). Its latest reaccreditation was approved on April 18, 2024.
In addition, it is a member of the Memorandum of Collaboration and Information Exchange between the Spanish Agency of Medicines and Health Products and the Ethics Committees for Research with Medicines.

Frequently Asked Questions
When should I submit a study?
Research projects must be submitted to the CEIm for evaluation before before of its realization. A research project cannot be initiated without the corresponding favorable opinion of the CEIm.
CEIm will not evaluate projects that have already been carried out.
When should I submit a study?
Organic Law 15/1999, of December 13, 1999, on the Protection of Personal Data has been repealed as of December 7, 2018. The law currently in force is Organic Law 3/2018, of December 5.
How should I submit documentation or notifications to CEIm?
All notifications and documents must be sent in digital format only. exclusively in digital format (Article 14.3 of Law 39/2015 of October 1) by email to the committee’s address(comité.etico.husa@saludcastillayleon.es) or to Clinical Trials with Medicinal Products, through the European Clinical Trials platform (CTIS).
No paper documentation will be accepted (Act 2021/04).
What documentation do I need to submit for my study?
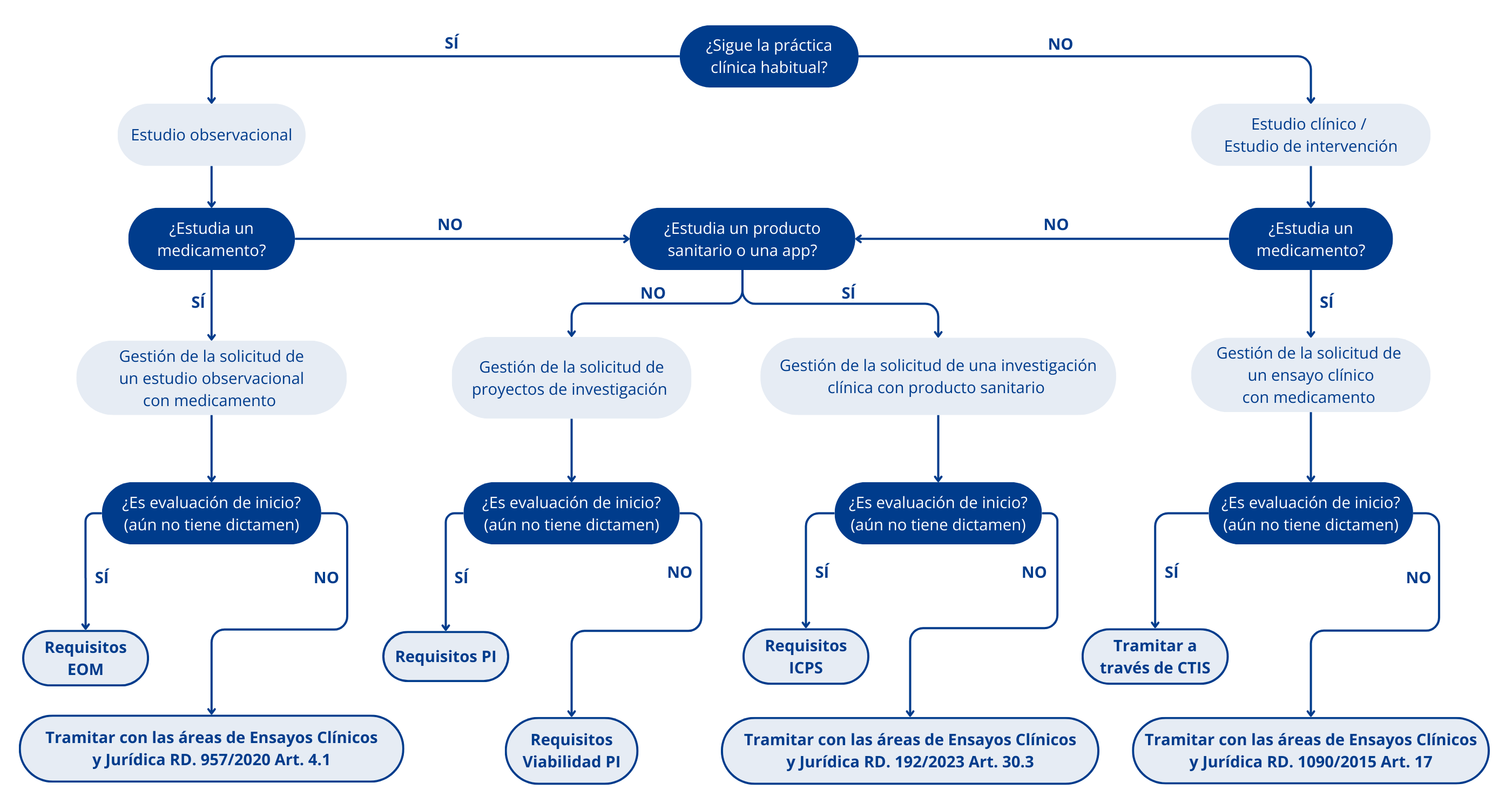
In the requirements section of this page you can find the documents to be submitted according to the type of study (see diagram above).
Is it necessary to add in the HIP the contact for the local IP?
The participant information sheet (HIP) should include the principal investigator’s contact information (name and telephone number) so that he/she can be contacted in case of questions.
In multicenter studies (or with a view to becoming so), it is ideal to leave a space for the center, the principal investigator and the corresponding contact telephone number to be added.
With whom should I manage the contract for a clinical research study?
To carry out the study in our Health Area you need to contact the legal services of the University Hospital of Salamanca(juridica.husa@saludcastillayleon.es) and the Biomedical Research Institute of Salamanca (ensayosclinicos@ibsal.es) to specify the legal and contractual aspects.
How do I know the classification of my study and the administrative route to follow?
The decision tree in the previous section can help you follow the appropriate administrative path.
How are the fees for the evaluation of studies processed?
For initial clinical trial evaluation submissions with drug as the reference IRB, the fee is unique for the agency and the committee. The payment of fees will be processed through this form.
The payment of fees for the evaluation of observational studies with drugs or clinical research with medical devices is done through form 046, for more information click on the following links:
- https://tributos.jcyl.es/web/jcyl/Tributos/es/Plantilla100/1284270178029/_/_/_
- https://tributos.jcyl.es/web/jcyl/Tributos/es/Plantilla100/1284270176021/_/_/_
The rate has an identification code:
HEALTH, D.G. Public Health, 306.1.0 Fee for sanitary services:
306.1.0.9: Biomedical and Health Sciences Research: For the processing and management of actions prior to the issuance of opinions by the Clinical Research Ethics Committee regarding the performance of clinical trials and post-authorization studies with drugs for human use or medical devices.
Do we accept opinions from another reference IRB/IEC?
For clinical drug trials:
– A clinical trial with medicinal product that has the favorable opinion of another reference CEIm would be under the protection of Article 17.2.a of Royal Decree 1090/2015 of December 4, which indicates that the opinion issued by a CEIm of national territory will be unique and binding, therefore, its evaluation by the Ethics Committee of the Salamanca Health Area is not necessary.
For observational studies:
– An observational study with a drug that has a favorable opinion from another reference IRB would be covered by article 4.1 of R.D. 957/2020 of November 3, which indicates that the opinion issued by an IRB in the national territory will be unique and binding; therefore, its evaluation by the Ethics Committee of the Salamanca Health Area is not necessary.
For clinical research with medical devices:
– A clinical research study with a medical device that has the favorable opinion of another reference CEIm would be covered by article 30.3 of Royal Decree 192/2023 of March 21, which indicates that the opinion issued by a CEIm of national territory will be unique and binding, therefore, it is not necessary for it to be evaluated by the Ethics Committee of the Salamanca Health Area.
For research projects:
– For research projects with a favorable opinion from another reference CEIm, local aspects will be evaluated to report on the feasibility of carrying out the study in our health area.